In this article, I will explain what are amino acids and why they are often called the building blocks of proteins. I will also present an amino acids chart to give a clear picture of their classification and types. Finally, I will discuss the physical properties of amino acids that determine how they function in living organisms.
what are amino acids
Amino acids are the derivatives of carboxylic acids in which an α-, β-, γ-, etc. hydrogen has been replaced by an amino (—NH2) group. The simplest amino acid is amino acetic compound which is generally known as glycine.
H2NCH2COOH
Glycine
Thus, amino acids are bifunctional compounds. However, the number of either functional group may be more than the other. The amino acids having more amino groups than carboxyl group are called basic Amino acids, and those having more carboxyl groups than amino groups are called acidic amino acids.
Amino acids are the structural units of proteins which are one of the three basic classes of foods: the other two are carbohydrates and fats. Proteins occur in most cells of animal body: they also occur in plants. When hydrolysed, proteins yield a mixture of amino acids which are usually called the protein forming or proteinaceous amino acids. The number of different proteinaceous amino acids obtained from nearly all proteins is about twenty. These are show in Table ( A few other amino acids may be present in one or the other protein). Although all amino acids can be assigned systematic IUPAC names, The common names given in the table are acceptable for convenience.
Amino acids chart
An amino acids chart shows the 20 amino acids with their structures, properties, and classifications. It helps in quickly identifying which are essential, non-essential, polar, nonpolar, acidic, or basic. This chart is a simple guide for studying proteins and biochemistry.
| Name | Structure | Isoelectric Point |
| Glycine | H2N—CH2—COOH | 6.0 |
| Alanine |
H2N – CH – COOH
|
CH3
| 6.0 |
| Vaine (e) |
H2N – CH – COOH
| CH(CH3)2 | 6.0 |
| Leucine (e) |
H2N – C – COOH
| CH2CH (CH3)2 | 6.0 |
| isoleucine (e) |
H2N – CH – COOH
| CH3CHCH2CH3 | 6.0 |
| phenylalanine (e) |
H2N – CH – COOH
| CH2C6H5 | 5.5 |
| Proline | 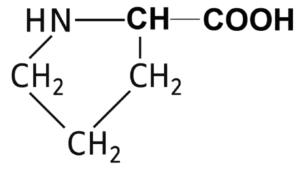 | 6.3 |
| Serine |
H2N – CH – COOH
| CH2OH | 5.7 |
| Threonine (e) |
H2N – CH– COOH
| HOCHCH3 | 5.6 |
| Tyrosine |
H2N – CH – COOH
| CH2C6H4OH | 5.7 |
| Glutamine |
H2N – CH – COOH
| CH2CH2CONH2 | 5.7 |
| Tryptophan (e) |
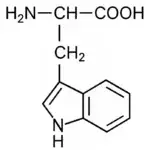
| 5.9 |
| Asparagine |
H2N – CH – COOH
| CH2CONH2 | 5.4 |
| cysteine |
H2N – CH – COOH
| CH2 SH | 5.0 |
| Methionine (e) |
H2N – CH – COOH
| CH2CH2 SCH3 | 5.7 |
| Aspartic Acid |
H2N – CH – COOH
| CH2COOH | 2.8 |
| Glutamic acid |
H2N – CH – COOH
| CH2CH2COOH | 3.2 |
| Lysin (e) |
H2N – CH – COOH
| (CH2)4 –NH2 | 9.7 |
| Ariginine (e) |
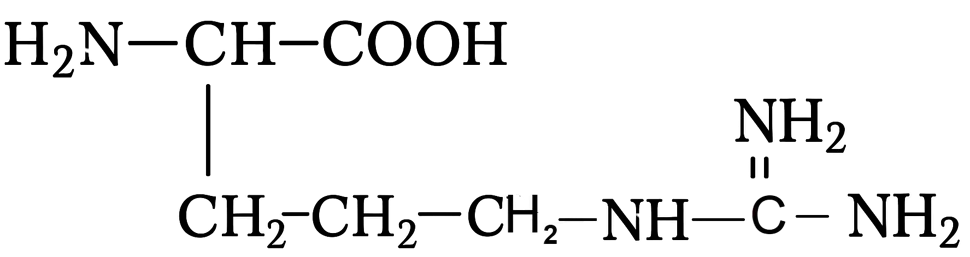
| 10.8 |
| Histidine (e) |
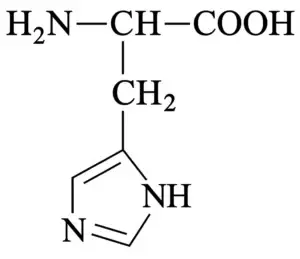 | 7.6 |
It can be seen from the table that all the proteinaceous amino acid are α- amino acids, and can be represented by the same basic structure, as shown below:
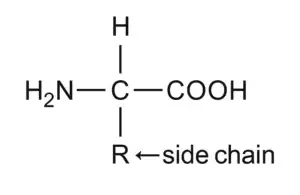
They differ from each other in the nature of the side chain (R) attached to the α-carbon atom. Except for glycine all the α-amino acids are chiral, and it has been found that all the naturally occurring α-amino acids belong to the L family of optical isomers, and have the S configuration at the α-carbon atom.
In proteins, the individual amino acids are joined by amide linkages, known as peptide bonds, as shown below:
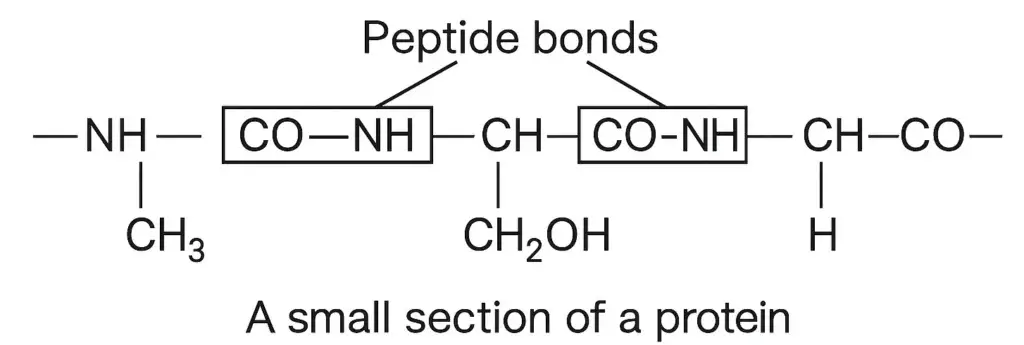
Of the twenty amino acids commonly found in proteins only ten can be synthesized in the animal body. The other ton cannot be synthesized in the body, and it becomes essential to supply them in food because a deficiency of any one of these will prevent the growth of the young animals, and may even cause their death. These are therefore known as essential amino acids. The essential amino acids have been designated by (e) after their names in Table.
Proteins that provide all the essential amino acids in about the proper proportion for human nutrition, are called complete proteins, e.g., those in meat, fish, egg and milk. Proteins that are deficient in one or more of the essential amino acids, are called incomplete proteins. Plant proteins are generally incomplete. For example, wheat, rice and corn are all deficient in lysine. Rice is also deficient in threonine, and corn is also deficient in tryptophan. Beans, peas, and other legumes contain the most complete proteins among the usual plants, but they lack methionine. Vegetarians can achieve an adequate intake of the essential amino acids if they eat foods from many different plants.
Physical Properties of Amino Acid
Amino acids are non-volatile crystalline solids that melt at fairly high temperature ( generally above 200°C), usually with decomposition. Their melting points are much higher than those of organic compounds of related structures and molecular weights. For example

Amino acids are fairly soluble in water. they are also soluble in highly polar organic solvents, but are insoluble in non-polar solvents. They generally have large dipole moments( glycine, µ = 14 D).
These properties are typical of salts and indicate that the basic amino and the acidic carboxyl groups of the same molecule neutralize each other to form an inner salt which is also known as a dipolar ion or a zwitterion, as shown below:
H₂N—CH₂—COOH ⇌ H₃N⁺—CH₂—COO⁻
Amino Acid polar ion if the amino acid
