The formula of sulfuric acid is H2SO4. It is a highly corrosive diprotic acid. Because it contains two acid protons. In simple words, it contains sulphate ions which are separate from the acidic H+ protons.
This acid requires extreme caution while using it, as it is extremely dangerous for your skin, teeth, eyes, and lungs. If used for a prolonged period, it can even be life-threatening.
The harmful effects of sulfuric acid are mostly found in people who are around it, such as factory workers. However, the extent of the damage can be determined by how much time they spend in the factory.
In the next paragraph, we will discuss the properties, structure, and uses of sulphuric acid in detail.
Used of sulfuric acid.
Did you know that sulfuric acid (H₂SO₄) is one of the most useful and important chemicals in the world. It is also called the “king of chemicals” because it is used in many industries. It helps in making fertilizer, which farmers use to grow crops. It is also used in the cleaning of metals and the production of fuel.
In short, sulfuric acid plays a very important role in both our daily lives and industrial work.
1. Sulfuric acid in Fertilizer Production.
The main use of sulfuric acid is in making fertilizers. It helps make ammonium sulfate and superphosphate, which add nutrients to the soil. These nutrients help plants grow faster and healthier. Almost half of all sulfuric acid made in the world is used for making fertilizers.
2. Iron and Steel Cleaning.
Sulfuric acid is used to clean iron and steel before they are used in factories. This process is called pickling. It helps remove rust and dirt from the metal surface. After cleaning, the metal becomes smooth and shiny, ready for painting or coating.
3. Making Other Chemicals.
Sulfuric acid is a very important raw material in many chemical factories. It is used to make other strong acids like phosphoric acid, nitric acid, and hydrochloric acid. It also helps in making soaps, dyes, and medicines that we use in everyday life.
4. Phosphoric Acid Production.
When sulfuric acid reacts with phosphate rock, it forms phosphoric acid. This new acid is very useful. It is used to make fertilizers, detergents, and even some food products.
5. Petroleum Industry.
In oil refineries, sulfuric acid is used in a process called alkylation. This process helps make high-quality petrol (gasoline). Sulfuric acid also helps remove unwanted materials from fuels, making them cleaner and better for use.
6. Cleaning and Maintenance.
A dilute form of sulfuric acid is used as a cleaning agent in many industries. It plays an important role in removing rust, grease, and stains from machines and pipelines. However, it must be used with great care. Because it is a strong acid.
7. Laboratory Use
Sulfuric acid is used extensively in many experiments in schools and industrial laboratories. It acts as a dehydrating agent, which means it removes water from other substances. It is also used to prepare various chemical solutions for experiments and research.
Physical Properties of Sulfuric acid.
- Chemical Formula Of Sulfuric acid : H₂SO₄
- Molar Mass of H2SO4: 98.079 g/mol
- Appearance: Colorless to slightly yellow, oily liquid
- Odor: Odorless
- Density of H2SO4: 1.84 g/cm³ (concentrated form)
- Boiling Point of H2SO4: 337 °C (639 °F)
- Melting Point of H2SO4 10 °C (50 °F)
- Solubility of H2SO4: Highly soluble in water (releases a lot of heat)
- Viscosity of H2SO4: Thick and oily
- Corrosiveness: Highly corrosive to metals and tissues.
Chemical Properties of Sulfuric acid.
- Strong Acid: Fully dissociates in water to produce H⁺ ions
- H₂SO₄→2H+ +SO₄ 2−
- Dehydrating Agent: Removes water from compounds (e.g., sugar becomes carbon)
- Oxidizing Agent: Can oxidize substances like carbon, sulfur, and metals
- Reacts with Bases: Forms salts (sulfates) and water
- H₂SO₄+2NaOH→Na₂SO₄+2H₂O
- Reacts with Metals: Produces hydrogen gas
- H₂SO₄+Zn→ZnSO₄+H₂↑
Role of Sulfuric Acid in Car Batteries.
You all know that car batteries are very important for vehicles. They store electrical energy that helps start the engine and power the lights, radio, and other systems. The primary chemical inside a battery is sulfuric acid (H₂SO₄). It plays a key role in helping the battery function properly.
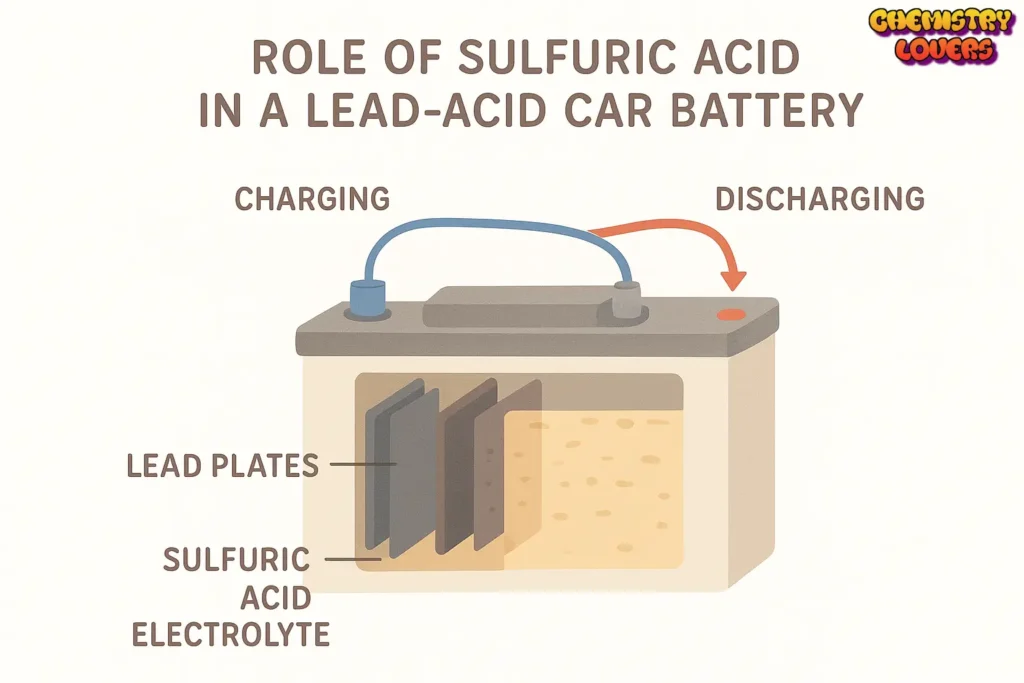
Why Sulfuric Acid Is Used in Car Batteries.
Sulfuric acid is used in batteries because it reacts easily with metals and produces electrical energy. It acts as an electrolyte, which means it helps the electric current flow between the battery plates. Without sulfuric acid, a battery can neither produce nor store electricity.
How It Works in the Battery.
Inside a car battery, there are two types of plates:
- Lead dioxide (PbO₂) — positive plate
- Sponge lead (Pb) — negative plate
Both plates are dipped in a mixture of sulfuric acid and water. When the battery is being used (discharging), a chemical reaction happens:
- Sulfuric acid reacts with the lead plates.
- This reaction produces electrons, which flow through wires and give energy to start the car.
When the battery is recharged, the reaction is reversed, and the sulfuric acid becomes strong again.
What Happens When the Battery Gets Weak.
When a car battery becomes old or discharged:
- The sulfuric acid turns into water, and its strength decreases.
- The voltage of the battery drops, and it cannot start the car easily.
That’s why mechanics check the acid level (specific gravity) of the battery using a hydrometer.
Safety and Handling.
Sulfuric acid is a strong and dangerous chemical. It can burn skin and damage clothing. Always follow safety tips:
- Do not touch the acid in the battery directly.
- Wear gloves and goggles to protect your eyes when checking or refilling.
- If it spills, clean it immediately with water and baking soda.
Also Read.
- Chelates – Definition, Types, and Uses
- Metallic Solids: Properties, Bonding, and Theories Explained
- Reversible and Irreversible Reactions Explained Simply
Sulfuric Acid (H₂SO₄) – Lewis Structure
The Lewis structure of sulfuric acid (H₂SO₄) shows a central sulfur atom bonded to two double-bonded oxygen atoms and two hydroxyl groups (–OH). These hydroxyl groups can donate hydrogen ions (H⁺), making H₂SO₄ a strong diprotic acid. The geometry around sulfur is approximately tetrahedral.
FAQs
1. What to do if H₂SO₄ gets on skin?
If a chemical gets on someone’s skin or gets into their eyes, rinse them thoroughly with water for at least fifteen to twenty minutes. If the chemical is swallowed, give the person plenty of water and milk immediately. If the condition worsens, consult a doctor.
2. Is H2SO4 a strong or weak acid?
Sulfuric acid is a strong acid. It completely dissociates in water, which is a sign of being a strong acid.
3. Who is the king of acids?
Sulfuric acid is called the king of acids.
