The Crystalline solids in which metal atoms are held together by metallic bonds are known as metallic solids.
Metallic Bond:
The force of attraction that binds positive metal ion to the number of electron within its sphere of influence is called metallic bond.
Theories of Metallic bonding:
Various Theories have been put forward to explain the properties of these type of crystals:
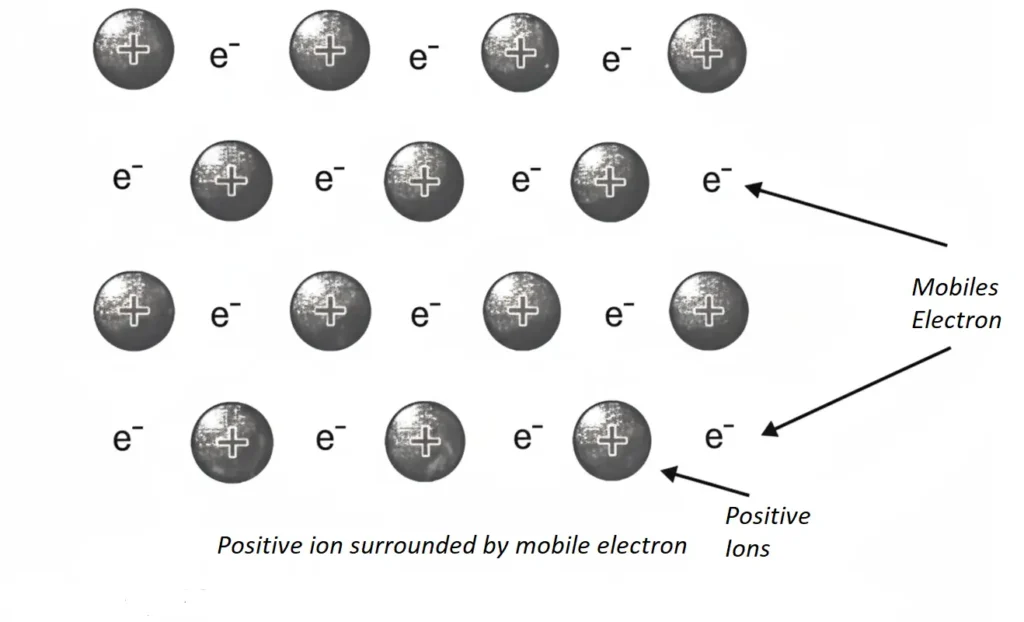
Electron pool or electron gas theory: This was proposed by Drude and extended by Loren in 1923, According to this theory, each atom present in a metallic crystal loses all its valence electrons. In this way a pool of electrons is formed. Fig. It is believed that positively charged metal ions are held together by pool of electrons. These positive ions are located at definite positions. They are situated at measurable distances from each other in the metallic crystal. The valence electrons are not associated with any individual ion. They move freely from one part to the other in the crystal lattice. following figure indicates positive ions surrounded by free electron.
Valence bond theory: This theory was given by L. Pauling. According to this theory, metallic bond behaves just like covalent bond. Covalent bonds are not localized. These are highly delocalized in metallic crystals. The delocalized electrons can explain the electrical and thermal conductivities.
Molecular orbital theory: This theory has recently been applied to explain the properties of metallic crystals, According to this theory, the electrons which are in the completely filled orbitals are localized. The atomic orbitals having valence electrons overlap to form a set of delocalized orbitals known as molecular orbitals. These are extended all over the crystal lattice. Due to such type of combination of atomic orbitals a large number of closely spaced states are formed called bands of energy. So this is also called as band theory. The properties of metallic solids depend upon the gap between two bands.
Properties of Metallic Solids:
Electrical conductivity: Metals are good conductors of electricity. The reason is that when electric field is applied, mobile electrons start moving towards positive pole. Hence a new electron coming from negative pole finds the space.
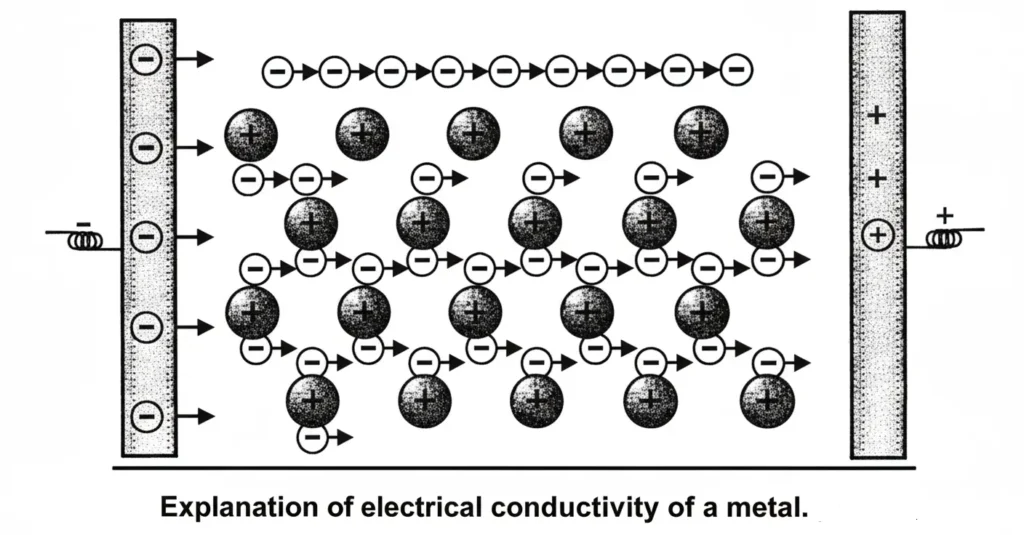
Effect of temperature on electrical conductivity: Sometimes when temperature increases conductivity of metals decreases. The reason is that the temperature causes positive ions to oscillate. This hinders free motion of mobile electrons among the positive ions. So electrical conductivity of metals decreases.
Thermal conductivity: If one end of a piece of metal is heated, the free electrons at one end absorbs heat energy. These electrons then move towards other end through the metallic lattice. In this process, they also collide with adjacent electrons and transfer them their energy.
Metallic luster: Most of the metals when freshly cut, show metallic luster, They show shining surfaces.
Reason: When light falls on the surface of metal, it collides with the free electrons and electrons show excitation from lower energy to higher energy level. Then these electrons come back to lower energy state and emit energy in the form of light. Due to this light, the metals look shining.
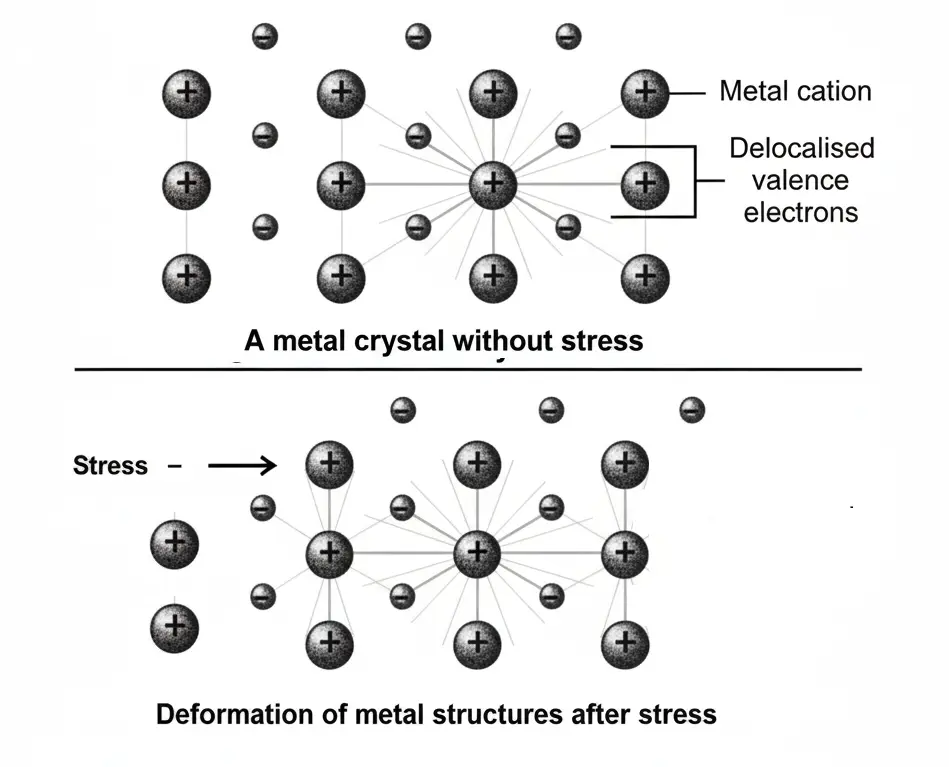
Malleable and ductile: Metals are malleable and ductile. The reason is that when stress is applied on metal surface, its layers slide past over one another and structure is changed as shown in Fig.
Metallic Solids — FAQ
What is a metallic solid?
A metallic solid is composed of metal atoms held together by metallic bonding — a lattice of positive ions surrounded by a delocalized sea of electrons.
What is metallic bonding and how does it work?
Metallic bonding arises when metal atoms donate valence electrons to a shared “sea” of delocalized electrons, binding the positive ions together.
What properties are characteristic of metallic solids?
They typically have high electrical and thermal conductivity, malleability, ductility, metallic luster, and variable melting points.
Why do metallic solids conduct electricity so well?
The delocalized electrons move freely under an electric field, carrying charge across the solid.
What are alloys and how do they change metallic properties?
Alloys are mixtures of metals (and sometimes nonmetals). They modify strength, corrosion resistance, conductivity, and melting point. Example: steel is stronger than pure iron.
