Diffusion and effusion are two important concepts in chemistry that explain how gases move and spread. Both processes involve the motion of gas molecules, but they occur in different ways. In this article, we will discuss the definitions of diffusion and effusion, highlight the key differences between them, and explain the formulas that describe these processes. By the end, you’ll have a clear understanding of how gases behave in open spaces as well as when passing through tiny openings.
There are two definition of Diffusion and effusion :
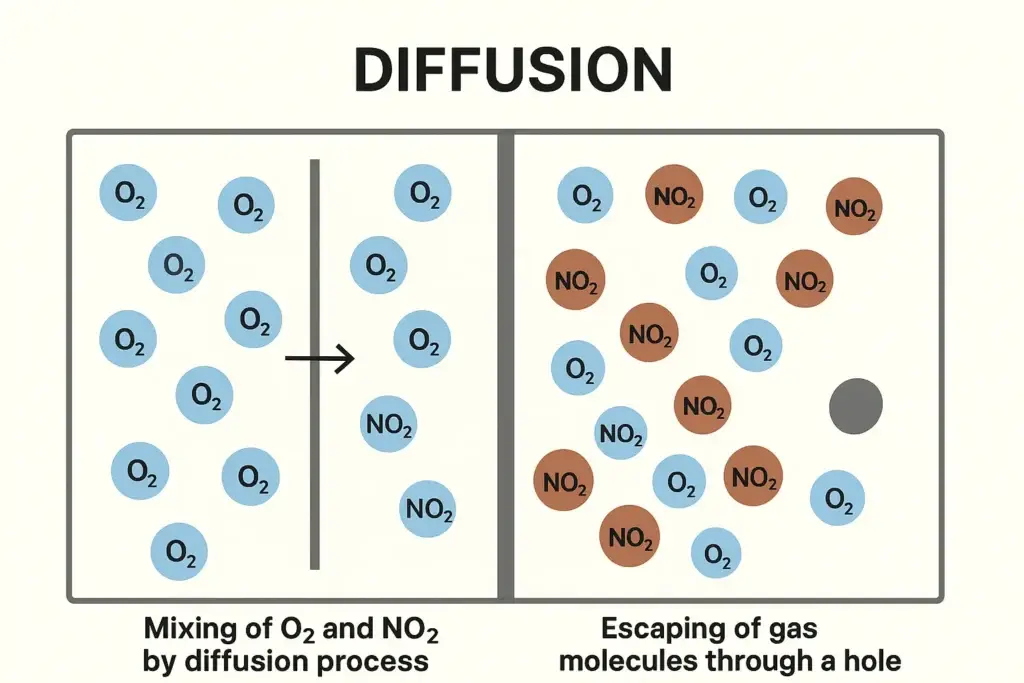
- The spontaneous mixing of molecules of different gases by random motion and collisions to form a homogeneous mixture is called diffusion.
- Diffusion is the spontaneous flow of molecules from an area of high concentration to an area of low concentration.
Example.
- The spreading of fragrance of a rose or a scent is due to diffusion.
- If two gases, say NO₂ and O₂, are divided by a partition, and when it is removed, they diffuse into one another. they generate a homogeneous mixture.
Effusion Definition:
- The passage of gas molecules one by one without collisions through a pin hole in their container into an evacuated space is called effusion.
- The escape of gas molecules one by one without collision through a very small hole of molecular dimension into a region of low pressure is called effusion.
Explanation:
when a gas is enclosed in a vessel and its molecules are allowed to spread through a very small hole into a vacuum, then it is called effusion. This spreading of molecules is not due to collisions. Actually, they have tendency to escape one by one
The reality is that the molecules of the gas are habitual in colliding with the walls of the vessel. When they reach the well and find a hole in that then they pass through the hole.
Difference between diffusion and effusion
| Diffusion | Effusion |
| Intermixing of molecules of different gases with one another at a particular temperature and pressure. | It is the movement of gas molecules under pressure through a tiny opening, having a diameter smaller than the mean free path of the gas molecules |
| The spreading and mixing of molecules is due to collisions. | The spreading of molecules is not due to collisions, but due to their tendency to escape one by one. |
| The molecules move from an area of high concentration to an area of low concentration. | The molecules travel from a region of high pressure to a region of low pressure. |
| Two or more than two substances (gases) are involved in mixing. | Only one substance (gas) is involved. |
| It may take place in gases, liquids as well as in solids, although slowly. | It takes place only in gases. |
| Example: Mixing and spreading of perfume molecules with air in a large room. | Examples: The coming out of hydrogen gas from rubber balloons within six to seven hours through a small opening. |
| Uses/applications: Separation of gases like hydrogen and deuterium. In dialysis as a mean of separating colloids from crystalloids | Uses/applications: Investigating low vapour pressure. Molecular weight determination. |
Diffusion and effusion formula
Here are the formulas for diffusion and effusion in simple form:
1. Diffusion Formula (Graham’s Law of Diffusion):
The rate of diffusion of a gas is inversely proportional to the square root of its molar mass.
\frac{r_1}{r_2} = \sqrt{\frac{M_2}{M_1}}
- r1,r2 = rates of diffusion of gases 1 and 2
- M1,M2 = molar masses of gases 1 and 2
2. Effusion Formula (Graham’s Law of Effusion):
The rate of effusion of a gas through a tiny hole (into vacuum) is also inversely proportional to the square root of its molar mass.
\frac{r_1}{r_2} = \sqrt{\frac{M_2}{M_1}}
Same formula as diffusion, but here the process is through a small hole without collisions (vacuum on the other side).