When a polydentate ligand surrounds the central metal atom or ion, cycle co-ordination complex ions are produced. These are called chelates.
When all the donor atoms of the polydentate ligands are coordinated with the same metal ion, then a complex is produced. This complex has one or more rings in its structure. This complex containing the ring is called chelated complex.
Example:
Pt+2 has coordination number 4. Four ligands can give their electron pairs. Oxalate ion is a bidentate ligand. One oxalate ion can give its two electron pairs. So two oxalate ions can give four electron pairs. Following diagrams makes the idea clear.
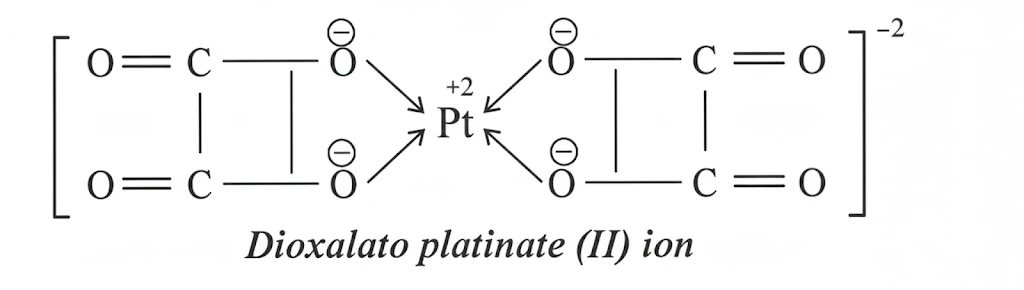
Two five membered rings are produced to give the complex ion. Thus complex is called chelate.
Chelating ligands:
The complexes which are given by polydentate ligands to the central metal ion are called chelates or chelated complexes. For this reason the polydentate ligand like oxalate ion are called chelating ligands.
Chelates uses:
Chelates are very useful compounds because they can hold metal ions tightly and make them more stable, soluble, and safe to use. They are used in medicine, industry, agriculture, and laboratories.
| Field | Example | Uses |
| Medicine | EDTA, DMSA | Remove toxic metals |
| Biology | Hemoglobin, Chlorophyll | Transport oxygen, photosynthesis |
| Agriculture | Fe-EDTA, Zn-EDTA | Chelated fertilizers |
| Industry | EDTA in detergents | Water softening, cleaning |
| Lab work | EDTA titration | Test water hardness |
| Environment | EDTA, NTA | Metal removal from waste water |
Types of Chelates:
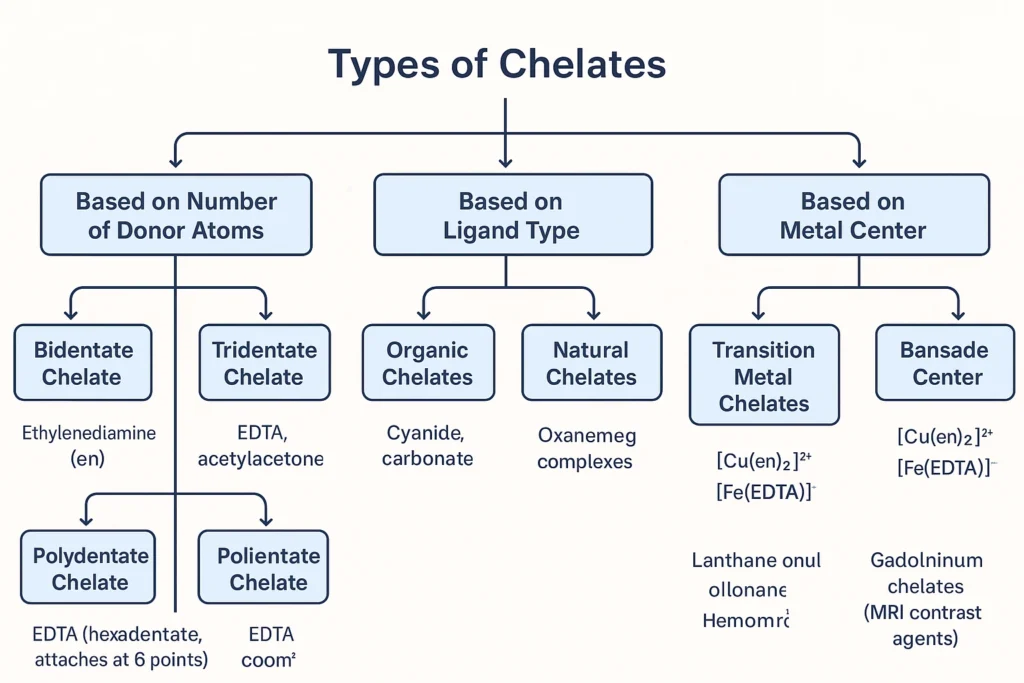
Chelates can be divided into different types based on how they are formed, what ligands they have, or where they are used. Let’s learn all the main types of chelates.
1. Based on Number of Donor Atoms (Denticity)
Chelates are formed by ligands — molecules that attach to a metal ion. The number of points (atoms) a ligand uses to attach is called denticity.
| Type | Description | Example |
| Bidentate Chelate | Ligand attaches to the metal at two points. | Ethylenediamine (en) forms [Ni(en)₃]²⁺ |
| Tridentate Chelate | Ligand attaches at three points. | Diethylenetriamine (dien) |
| Polydentate Chelate | Ligand attaches at four or more points. | EDTA (hexadentate, attaches at 6 points) |
Remember: More attachment points = stronger and more stable chelate.
2. Based on Ligand Type.
| Type | Description | Example |
| Organic Chelates | Made with carbon-based ligands. | EDTA, acetylacetone |
| Inorganic Chelates | Made with inorganic ligands. | Cyanide, carbonate |
| Natural Chelates | Found in living things. | Hemoglobin (Fe²⁺), Chlorophyll (Mg²⁺), Vitamin B₁₂ (Co³⁺) |
| Synthetic Chelates | Man-made compounds used in labs or industries. | NTA, DTPA, EGTA |
3. Based on Ring Size.
Chelates form ring-like structures when ligands bond to metals.
| Type | Description | Example |
| Five-membered ring chelates | Common and very stable | Oxalate complex with Fe²⁺ |
| Six-membered ring chelates | Also quite stable | Ethylenediamine complexes |
Smaller or larger rings are usually less stable.
4. Based on Metal Center.
| Type | Description | Example |
| Transition Metal Chelates | Most common; involve d-block metals | [Cu(en)₂]²⁺, [Fe(EDTA)]⁻ |
| Main Group Metal Chelates | Formed with s- or p-block metals | [Ca(EDTA)]²⁻, [Mg(oxalate)] |
| Lanthanide and Actinide Chelates | Used in nuclear and imaging studies | Gadolinium chelates (MRI contrast agents) |
Chelates Side Effects.
Chelates are helpful, but using too much can be harmful.
Common Side Effects
- Stomach pain or vomiting
- Headache or tiredness
- Loss of appetite
- Mild fever
Serious Side Effects.
- Low calcium in blood
- Kidney damage
- Liver problems
- Skin allergy or rash
- Loss of good minerals (like zinc, iron)
Chelates are very useful compounds that help in medicine, industry, and agriculture. But using them too much or in the wrong way can cause side effects. Always use chelates carefully and under expert guidance.
Chelate properties.
- Very stable compounds.
- Often colored (especially with transition metals).
- Less reactive than simple salts.
- Used in water softening and titrations.
- Soluble in water or organic solvents depending on the ligand.
