About a very important concept in Organic Chemistry called Inductive Effect I would like to briefly present. The Inductive Effect is simply the shifting of electrons in a molecule because of differences in atomic electronegativity ratios between atoms.
This is known as being universal: Each atom in a molecule has a desire for electron strength of its own.When one atom grabs an electron a little more than the other, it creates a small positive or negative charge as a result of this process.That pulling or pushing of electrons through sigma bonds is called the Inductive Effect.
Example.
For example, let’s take this.
Chloroethane (CH₃–CH₂–Cl)
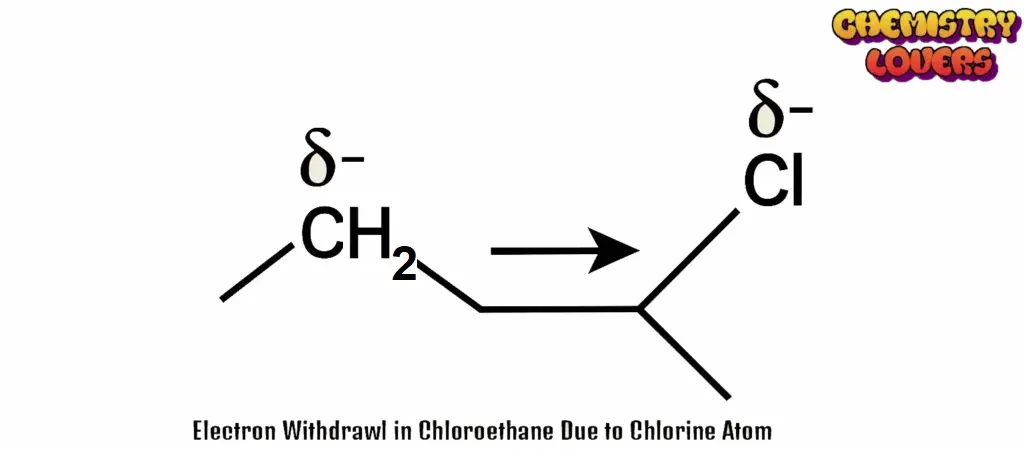
In this molecule, chlorine (Cl) is more electronegative than carbon.
This makes chlorine slightly negative (–) and the carbon next to it slightly positive (+).
This charge effect then slowly decreases along the chain — it weakens as you move away from the chlorine atom.
Definaition Of Inductive Effect.
Inductive Effect is the electron-withdrawing or electron-donating effect transmitted through sigma bonds in a carbon chain, due to differences in electronegativity of atoms.
Types of Inductive Effect.
There are two main types of Inductive Effect:
–I Effect (Negative Inductive Effect).
When an atom or group pulls electrons towards itself, it is called the –I Effect.
Examples of –I groups
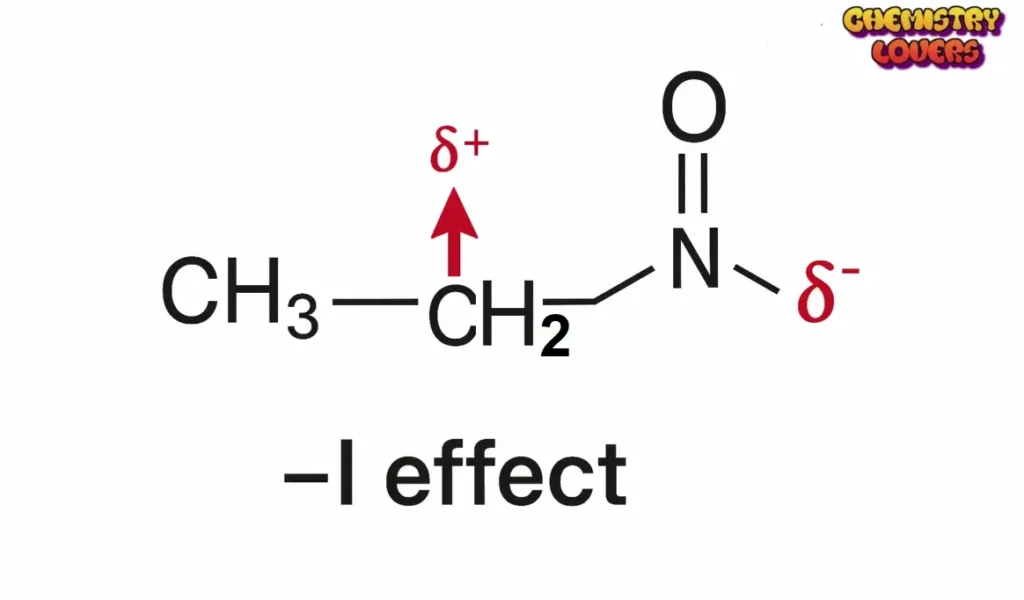
- –NO₂ (Nitro)
- –Cl, –Br, –I (Halogens)
- –COOH (Carboxyl)
- –CN (Cyano)
- –CHO (Aldehyde)
These groups make the carbon atom slightly positive by taking away some electron density.
+I Effect (Positive Inductive Effect).
When a group pushes electrons away from itself toward other atoms, it is called the +I Effect.
Examples of +I groups:
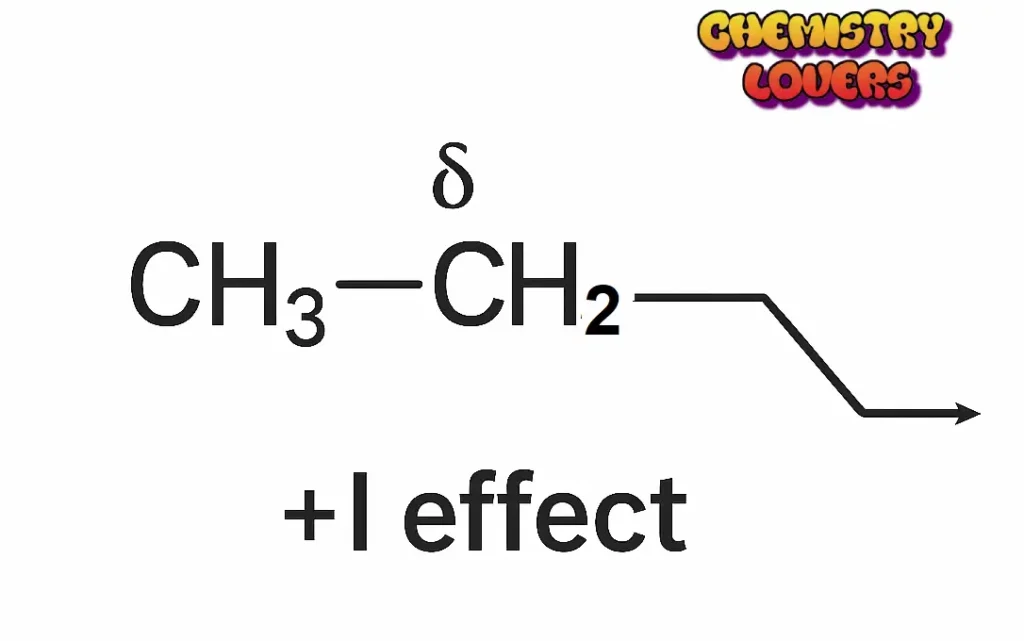
- –CH₃ (Methyl)
- –C₂H₅ (Ethyl)
- –(CH₃)₃C (Tertiary butyl)
- Metal atoms like Na, K, Mg also show +I effect
These groups make the nearby carbon more negative by donating electrons.
Order of Decrease.
The inductive effect becomes weaker as the distance from the group increases.
For example:
In CH₃–CH₂–CH₂–Cl
→ The carbon near Cl is affected the most,
→ The next carbon is affected less,
→ And the farthest carbon is almost not affected.
Importance of Inductive Effect.
The inductive effect helps us understand many things in organic chemistry:
- Acid Strength: –I groups increase acid strength by pulling electrons (making the molecule more acidic).Example: Cl–CH₂–COOH is more acidic than CH₃–COOH.
- Basic Strength: +I groups increase basic strength by donating electrons.Example: (CH₃)₂NH is more basic than NH₃.
- Reactivity of Molecules: It explains why some compounds react faster than others.
- Polarity and Stability: Molecules become polar due to electron shifting, which affects their stability and solubility.
Difference Between inductive effect and electromeric effect.
The Electromeric Effect is a temporary effect.
It happens only when a reagent (like an acid or base) attacks a molecule that has a double bond or pi bond.
In this case, the shared electrons move completely from one atom to another.
Example:
When ethene (CH₂=CH₂) reacts with H⁺, the pi electrons move towards one carbon.
This happens only during the reaction — after that, electrons go back to normal.
| Feature | inductive effect | Electromeric Effect |
| Type of Bond | Sigma bond (single bond) | Pi bond (double bond) |
| Nature | Permanent | Temporary |
| Cause | Difference in electronegativity | Presence of attacking reagent |
| Electron Movement | Partial shift of electrons | Complete transfer of electrons |
| Example | CH₃–CH₂–Cl | CH₂=CH₂ reacting with H⁺ |
In short:
Inductive Effect = always active, small shift
Electromeric Effect = happens only during reaction, big shift
Difference Between inductive effect and resonance effect.
The Resonance Effect happens when electrons move between double bonds and lone pairs in a molecule.
It gives multiple possible structures called resonance structures.
The actual molecule is a mix of all these structures.
Example: In benzene, the double bonds keep shifting — so, all carbon–carbon bonds become equal.
| Feature | inductive effect | Resonance effect |
| Type of Bond | Sigma bond | Pi bond |
| Nature | Permanent | Permanent |
| Electron Movement | Through sigma bond | Through pi bond and lone pairs |
| Range | Weak, short-range effect | Strong, delocalized over the molecule |
| Example | CH₃–CH₂–Cl | Benzene ring (C₆H₆) |
Summary.
- Inductive Effect → Small electron pull or push through sigma bonds.
- Electromeric Effect → Big temporary shift during reaction through pi bonds.
- Resonance Effect → Continuous sharing of electrons in molecules with double bonds.
All three explain how electrons move, but each works in a different way and different situation.
