In this experiment, I will purify commercial NaCl by passing HCl gas through its saturated solution. The process is based on the common ion effect, which decreases the solubility of NaCl and allows pure crystals to separate from the impure sample.
Apparatus and Chemicals.
| Apparatus | Chemicals |
| Beaker 500 cm3 | Commercial sodium chloride (NaCl) |
| Funnel ( for filtration) | Hydrogen chloride gas (HCl) (dry) |
| Round-bottom flask | Distilled or deionized water |
| Burner | Activated charcoal (optional) |
| Glass tubing | Dilute sodium hydroxide solution (NaOH) |
| Filter paper | Ethanol (absolute or rectified) (optional) |
| Stand with clamp (for holding gas tube) | Indicator paper (pH paper) |
| Evaporating dish or crystallizing dish | – |
| pH paper or pH meter | – |
Theory.
Common salt contains impurities like CaCl2, MgCl2. Pure salt can be obtained by passing HCI gas. NaCl on dissolving in water splits into its respective ions.
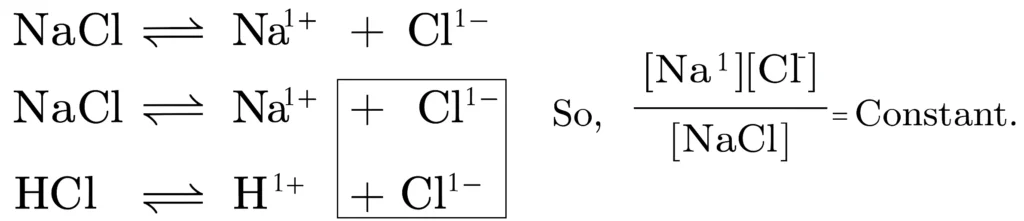
The molar concentration of chloride ion increases in the presence of HCI in H2O due to common ion effect. Due to increase in the number of chloride ions, the solubility product of Na1+ and CI1- becomes greater therefore the NaCl, separates out as crystals.
Solubility product of any substance is defined as the product of the molar concentrations of its ions in equilibrium with the solid salt in its saturated solution at a given temperature. The solubility products of MgCl2 and CaCl2 are high as compared to NaCl, so they are not precipitated.
Procedure.
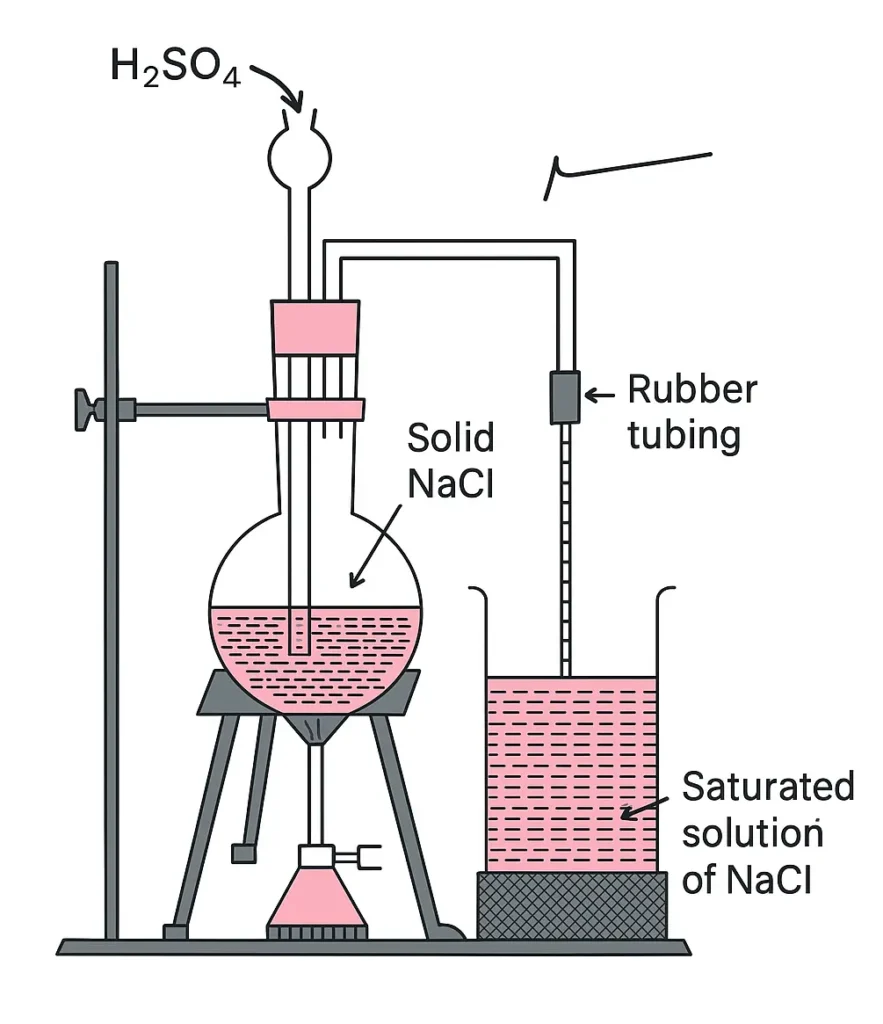
Take about 75 cm3 of distilled water in a 100 cm3 beaker. Add pinch by pinch the given sample of commercial NaCl with constant stirring till some of it remains undissolved. Filter this saturated solution in another beaker. Now prepare HCI gas by heating conc. H2SO4 with NaCI placed in a round-bottom flask. Pass HCI gas through saturated solution of NaCl as shown in the picture. When NaCI precipitates out, filter the solution. Pure NaCl can be dried by pressing gently between the folds of the filter paper.
Safety Tips.
- Gloves, lab coats, and goggles must be worn to protect against chemical splashes.
- HCl gas is toxic and irritating, never inhale it.
- If gas or acid comes into contact with skin, wash immediately with water.
- Avoid eating or drinking in the lab and do not touch your mouth or eyes while working.
