In this article, I will explain what is a salt bridge, Function of salt bridge, Voltaic cell as a reversible cell, Salt Bridge Amino Acids, Difference between electrolytic and galvaic cell, and how it affects the performance of your system.
A salt bridge is a device used in electrochemical cells that helps connect two half-cells and allows the flow of ions, Maintaining electrical neutrality and allowing the solution to mix directly helps complete the circuit.
It is an intermediate medium by means of which two electrolytic solutions are connected with each other. It is a U-shaped glass tube having a saturated solution of some strong electrolyte like KCl, K2SO4 or KNO3. The glass tube is sealed at both of its ends by a porous material like a glass wool or cotton plug. A jelly type material holds the solution in the U-tube.
This solution keeps the internal continuity of two half reaction taking place in 2 different half cells. It prevents the physical contact between the two electrolytic solutions. When the electrodes of the cell are connected, electrons flow from anode to the cathode. At the same time, anions move toward the anode compartment and cations move toward the cathode compartment.. In this way, the solution of two half-cells remains neutral.
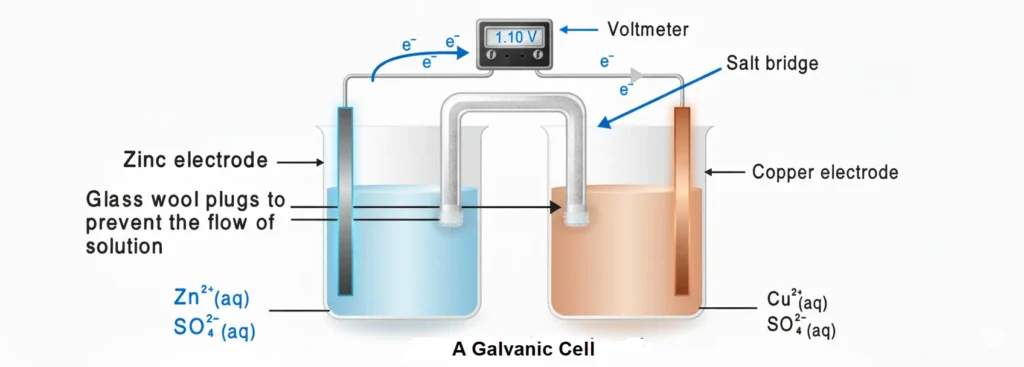
If diffusional exchange of ions does not take place, accumulation of the net charge in the beaker, flow of electrons from external circuit and oxidation reduction reaction will immediately stop.
Function of salt bridge:
it has two major function:
- It connects the solutions in two half cells and completes the cell circuit.
- It maintains the electrical neutrality by the diffusion of ions through it.
Voltaic cell as a reversible cell:
The voltaic cell can be changed into reversible cell. This is done by replacing the circuit of voltaic cell with a source of electricity which opposes the voltaic cell. The reactions occurring at electrodes can be reversed. Then the external source pf electricity will push the electrons in the opposite direction and supplies energy to the cell. In this way , a reverse non-spontaneous reaction takes place. This is known as a reversible cell.
Reversed Reaction:
At cathode:
\(\text{Zn}^{2+}_{(aq)} + 2e^- \;\longrightarrow\; \text{Zn}_{(s)}\)
(reduction)
At anode:
\(\text{Cu}_{(s)} \;\longrightarrow\; \text{Cu}^{2+}_{(aq)} + 2e^-\)
(oxidation)
Overall Reaction:
\(\text{Zn}^{2+}_{(aq)} + \text{Cu}_{(s)} \;\longrightarrow\; \text{Zn}_{(s)} + \text{Cu}^{2+}_{(aq)}\)
(Redox Reaction)
In the Reversed cell , oxidation takes place at copper electrode while reduction occurs at zinc electrode ( Cathode has changed to anode and vice versa). The cell will work as an electrolytic cell instead of voltaic or galvanic cell.
Difference between electrolytic and galvanic cell.
| Electrolytic cell | Galvanic cell |
| The Device in which electric energy is converted into chemical energy is called electrolytic cell. | The device in which chemical energy is converted into electric energy is called galvanic cell. |
| In this cell current is used to drive a chemical reaction. | In this cell current is produced as a result of chemical reaction. |
| Non-spontaneous oxidation reduction reactions take place. | Spontaneous oxidation-reduction reaction take place. |
| In this cell, electrolysis takes place. | In this cell electric conduction takes place. |
| Example: Down’s cell, Nelson’s cell | Example: Daniel’s cell, Fuel cell |
Also Read.
- Raoult’s Law – Definition, Formula & Graph
- Metallic Solids: Properties, Bonding, and Theories Explained
- Atomic Radius – Definition, variation, Trends, and Examples
Salt Bridge Amino Acids:
A salt bridge in amino acids is an ionic bond between a positively charged (basic) amino acid and a negatively charged (acidic) amino acid inside a protein.
Amino acids involved in salt bridges:
- Positively charged (basic) amino acids
- Lysine (Lys, K) → –NH₃⁺ group
- Arginine (Arg, R) → guanidinium group (–C(NH₂)₂⁺)
- Histidine (His, H) → imidazolium group (at acidic pH)
- Negatively charged (acidic) amino acids
- Aspartate (Asp, D) → –COO⁻ group
- Glutamate (Glu, E) → –COO⁻ group
Importance of Salt Bridges:
- Stabilize tertiary and quaternary structures of proteins.
- Help in enzyme active site geometry.
- Contribute to protein folding and binding specificity.
- Affect protein stability at different pH values (since ionization changes with pH).
