The vaporization of a solid directly on heating without passing through the liquid phase and condensation of the vapor on cooling to solid without passing through liquid phase is called sublimation.
Example.
The following are some important substances which can be sublimed.
- Naphthalene
- iodine
- Ammonium Chloride
- Benzoic acid
- Camphor
- Anthracene
- anthraquinone
- Hexachlororthane
Example of Sublimation in Daily Life:
- Dry ice (solid CO₂): Sublimes at room temperature, producing dense fog-like vapor.
- Naphthalene balls: Slowly disappear in cupboards due to sublimation.
- Iodine crystals: When heated, they directly form purple vapors.
- Camphor: Used in rituals and medicines, sublimes at room temperature.
Limitations:
It is a separation and purification technique. Only those compounds show this property which have high vapor pressure at a temperature below their melting point.
Examples of Limitations:
- Salt (NaCl): Cannot be purified by sublimation because it does not sublime.
- Sugar: Decomposes on heating instead of subliming.
- Copper Sulfate (CuSO₄): Dehydrates and changes color on heating rather than subliming.
- Large-scale purification: Sublimation is impractical for bulk materials like ores.
- High energy demand: Substances requiring very high sublimation temperatures are uneconomical to process.
Sublimand:
The solid substance being sublimed is called sublimand.
Common Examples of Sublimands:
Here are some well-known substances that act as sublimands:
- Iodine (I₂) → On heating, iodine crystals turn directly into purple vapor.
- Dry Ice (Solid CO₂) → Sublimes at room temperature without melting, producing dense white vapor.
- Naphthalene → Found in mothballs; sublimates slowly at room temperature.
- Camphor → Used in medicines and rituals; sublimates easily at normal conditions.
- Ammonium Chloride (NH₄Cl) → On heating, it sublimates to give ammonia and hydrogen chloride gases.
- Arsenic & Sulfur → Some allotropes of these elements can sublime on heating.
Sublimate:
The pure solid obtained after sublimation is called sublimate.
Examples of Sublimate:
- Iodine vapors (purple fumes → later condense into pure iodine crystals)
- Carbon dioxide gas from dry ice sublimation
- Camphor vapors
- Naphthalene vapors protecting clothes
- Pure ammonium chloride deposit after sublimation
Also Read.
- Raoult’s Law – Definition, Formula & Graph
- Metallic Solids: Properties, Bonding, and Theories Explained
- Atomic Radius – Definition, variation, Trends, and Examples
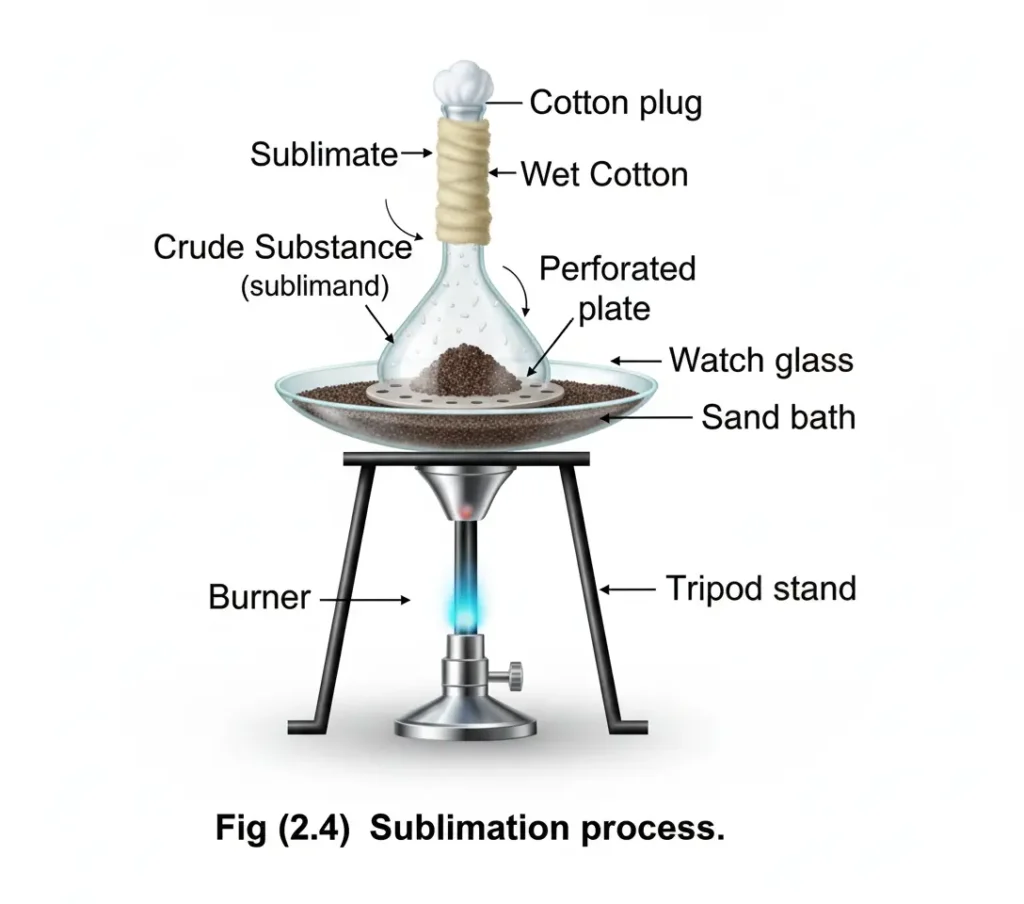
Process of sublimation:
In this process, a substance to be sublimed, is taken in a watch glass which is covered with an inverted funnel. Stem of funnel is closed with a cotton plug. Heat the substance slowly on the sand bath . At the same time cool the funnel with wet cotton. The compound sublimes and the pure solid deposits on the inner side of the funnel. in a better method, the process is carried out in a cold finger.
Cold Finger:
A cold finger is a glass tube filled with ice which is fitted into the top opening of the flask with a rubber adapter. The sample to be sublimed is put on the bottom of the flask. It is sublimed by heating and sublimate is deposited on chilled piece of cold finger which is just above the bottom of the flask. After completing the sublimation process, cold finger is taken out of the flask and sublimate is removed with the help of spatula.

